A breakthrough treatment for hepatitis B
... and four other medical breakthroughs this week
Before I start: I’ve been working with a team of doctors and AI specialists to build a platform for scientific research — it will guide you to produce a publication-quality systematic review, end to end, in <1 day. It’s made for researchers in academia, biotech or pharma. You can request a demo here, and we’ll show you what it can do!
1. Functional cure in hepatitis B
Hepatitis B affects more than 250,000,000 people globally, and causes about 60% of all liver cancers. The problem is getting bigger, not smaller: in 2050 it’s expected hepatitis B will cause 560,000 cases of liver cancer globally.
Hepatitis B is extremely difficult to cure because the virus forms DNA within liver cells (called closed circular DNA) and can integrate into the host genome — allowing it to produce new transcripts and sustain its replication. The current therapies (Entecavir, Tenofovir disoproxil fumarate and Tenofovir alafenamide, which were approved in 2005, 2008 and 2016, respectively) achieve functional cure in <0.5% of patients.
This concept of functional cure is important — its definition is ‘loss of the hepatitis B surface antigen (HBsAg) and undetectable hepatitis B DNA, for ≥24 weeks after treatment stops’. The goal of functional cure is to drive down the virus to a level that the immune system can control on its own.
This week, GSK (in partnership with Ionis Pharma) announced breakthrough data for two phase 3 trials, using Bepirovirsen, a once-weekly injectable antisense oligonucleotide that targets and degrades all 4 of the main hepatitis B transcripts (though some have argued it’s working via a different mechanism: stimulation of innate immunity).
The key caveat is that GSK haven’t actually told us how good the data are…. just that the study met its primary endpoints.
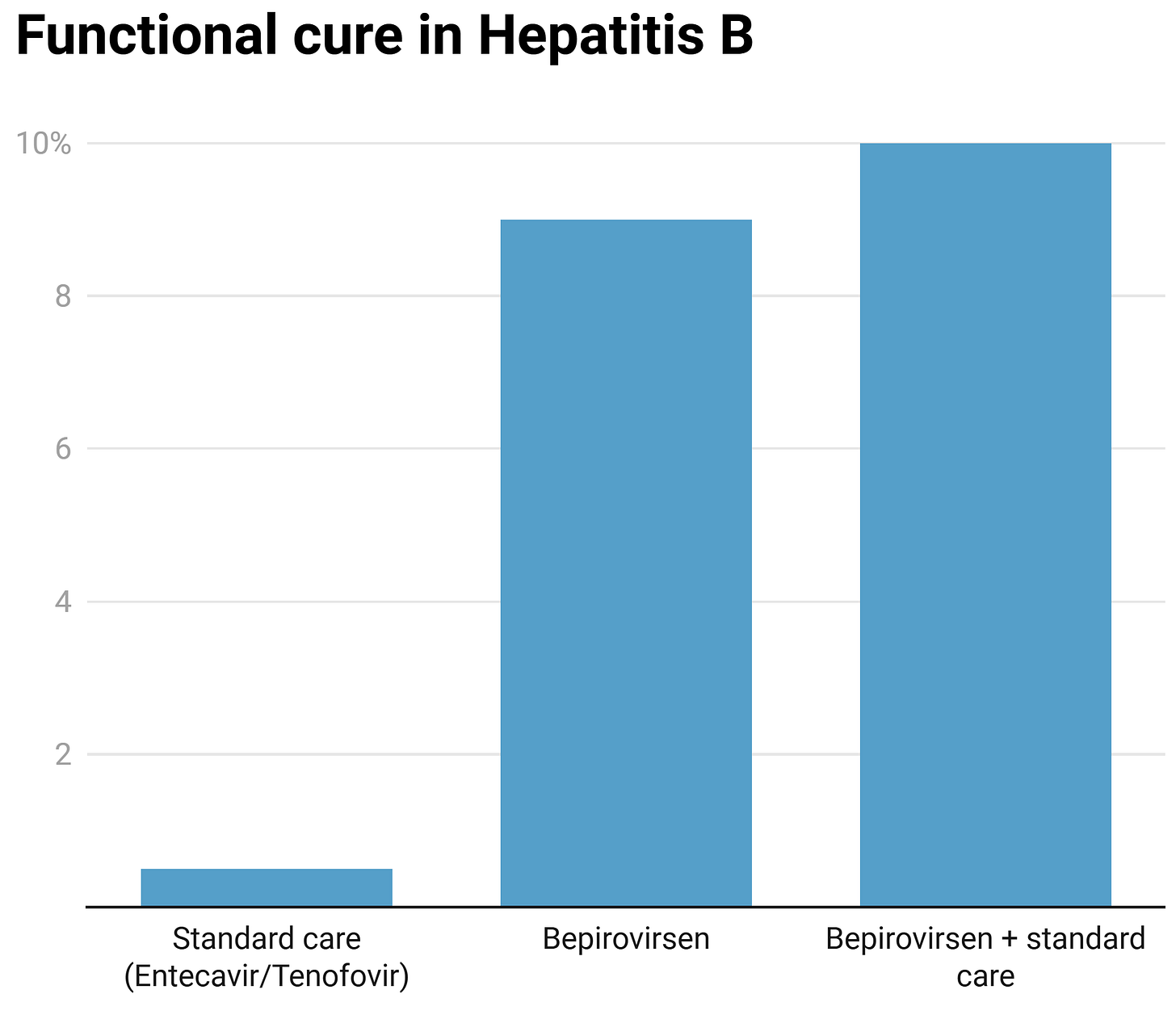
We can see the phase 2B data, though, where Bepirovirsen achieved functional cure of 9% on its own, and 10% when added to standard care (this 10% number is where experts agree would be a significant advance):
This is far from the only medicine being tested for Hepatitis B, too — there are more than 10 in clinical trials, including siRNAs, entry inhibitors, capsid inhibitors, therapeutic vaccines and even gene editing approaches!
2. Tirzepatide (Mounjaro) helps treat psoriatic arthritis
If you talk to people taking the GLP1/GIP receptor agonist, Tirzepatide, they might say they’ve seen improvement in conditions that seem unrelated to weight loss — like Crohn’s, ankylosing spondylitis, or psoriatic arthritis. What links all of these conditions is inflammation.
There have been no clinical trials to prove this, though — until now. This trial is in patients with overweight/obesity and psoriatic arthritis. Psoriatic arthritis is (usually) found in patients with the skin condition, psoriasis — it causes pain, swelling and potentially disabling joint deformation.
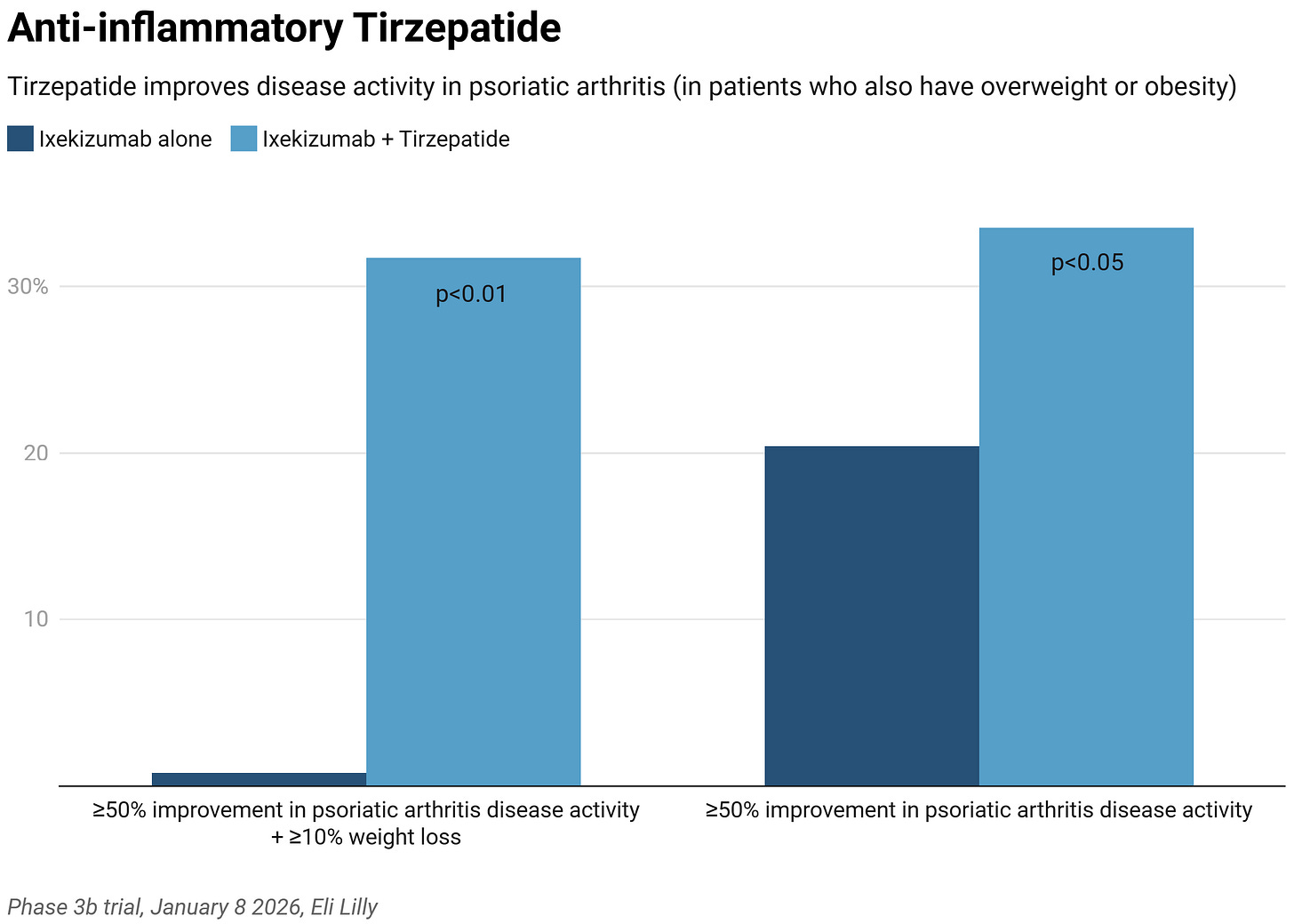
The trial tests an approved therapy alone (the IL17A inhibitor, Ixekizumab) vs. Ixekizumab in combination with Tirzepatide. 20.4% of patients had improvement in psoriatic arthritis disease activity in the Ixekizumab group vs. 33.5% in the combination group:
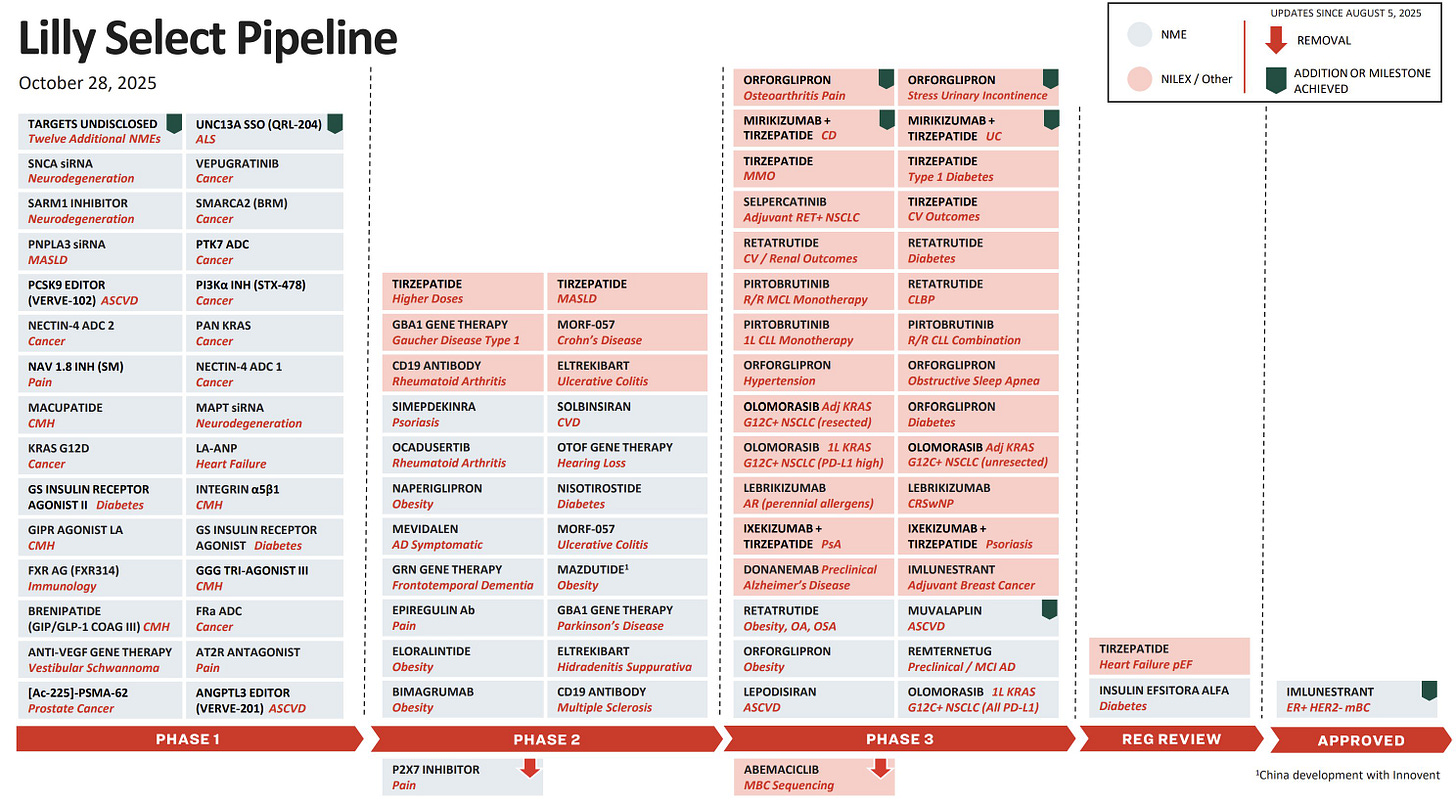
This is really only the beginning of this story: you can see from Eli Lilly’s pipeline that there are many more studies like this coming, leaning on the anti-inflammatory effects of GLP1s to help patients with Crohn’s, ulcerative colitis, and psoriasis.
3. T regulatory cells reduce cardiac inflammation
Last year the Nobel Prize in Medicine went to the discovery of T regulatory cells, which help our body to recognise itself — preventing autoimmunity and suppressing inflammation.
The key cytokine that controls T regulatory cells is interleukin 2 (IL2). At high doses it actually stimulates T killer cells (so it’s used in melanoma, for example), but at low doses it helps produce T regulatory cells.
Recently there have been successful(ish) trials using low-dose IL2 to suppress inflammation and improve outcomes in ALS and Behçet’s syndrome. This is a new trial that tests low-dose IL2 after a heart attack (acute coronary syndrome, specifically).
The trial is small and early (phase 2), but low-dose IL2 successfully boosted numbers of T regulatory cells and reduced arterial inflammation. In the two years following, 3 people in the standard care group had major cardiovascular events vs. 0 in the low-dose IL2 group:
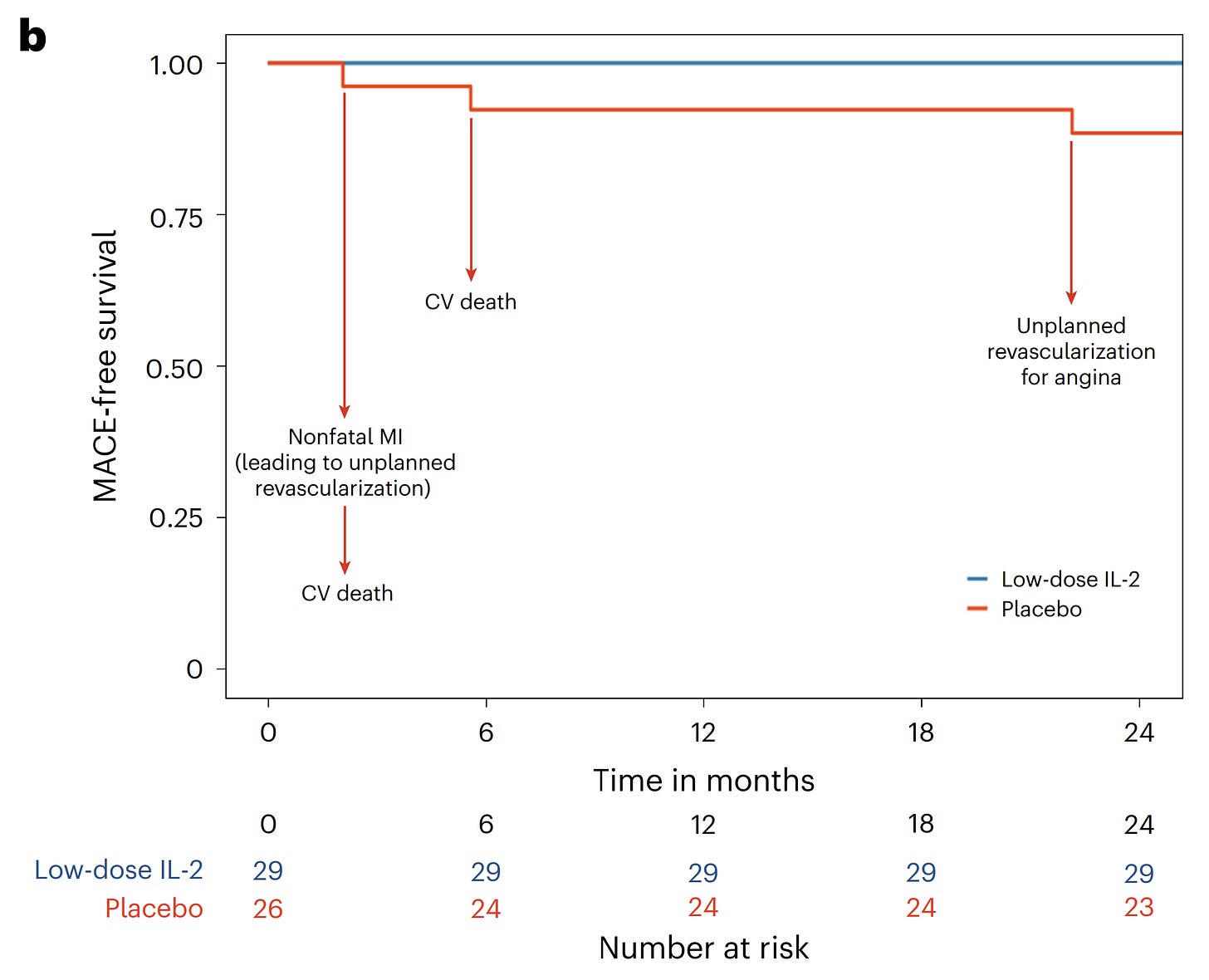
This field is rich with emerging potential indications: there are ongoing trials for low-dose IL2 and T regulatory cells in newly diagnosed type 1 diabetes, kidney transplant, and atopic dermatitis!
4. A novel anti-inflammatory
Low-dose IL2 is one way to suppress inflammation, but there’s many more where that came from! This figure nicely shows the key targets: