How the EBV virus causes multiple sclerosis
And 4 other medical breakthroughs this week
Before I start: if you’re doing research in an academic lab, in a hospital, in biotech, or in pharma, you might be interested in something I’ve been building with a team of doctors and AI engineers. It’s a scientific research platform, that guides you to produce a publication-quality systematic review, end to end, in <1 day. You can see more information, sign up, and request a demo here!
1. How EBV causes multiple sclerosis
Multiple sclerosis (MS) is an autoimmune neurological condition. There are some known risk factors: female sex, vitamin D deficiency, high latitude (Scotland, for example, is a hotspot).
The other factor that unifies almost everyone with MS is previous infection with the Epstein-Barr Virus (EBV). This is partly because of molecular mimicry: immune responses against an EBV protein (called EBNA1) cross-react with proteins in the brain and spinal cord, destroying myelin and affecting nerve conduction.
Almost all of us have had EBV, though, and MS only affects about 0.1-0.2% of people, so EBV alone can’t be the whole explanation. A trio of studies in this week’s Cell sheds light on this, with three key advances.
The first discovery: in people carrying a specific allele (HLA-DR15), EBV-infected B cells present antigens from the brain (like myelin) much more than non-carriers.
The second discovery: a specific EBV protein (called LMP-1) helps these B cells persist in the brain and spinal cord.
The third discovery: the autoimmune response is broader than we thought — including against the ion channel, ANO2 — which could help explain how MS spreads beyond myelin alone.
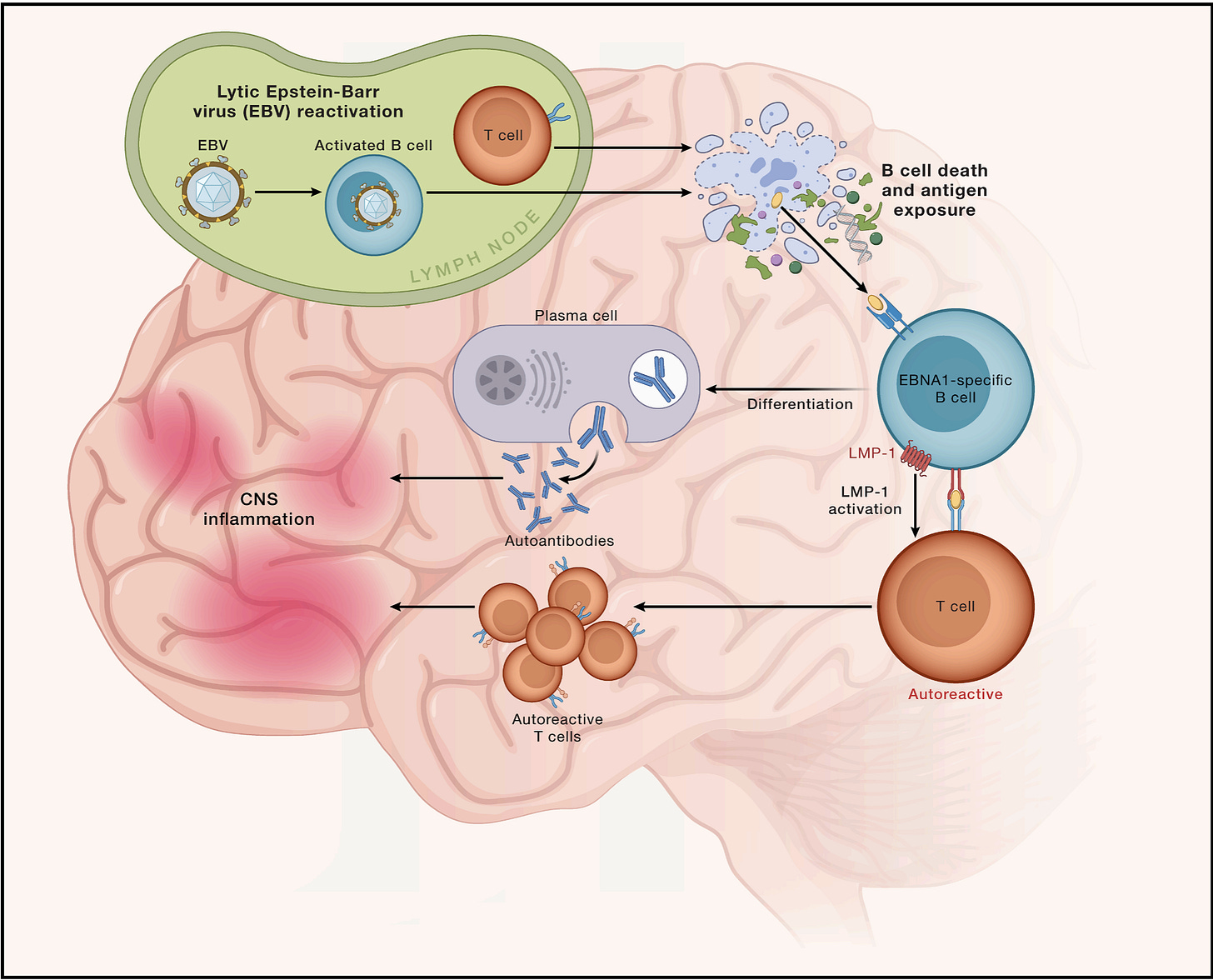
These discoveries could help identify people at higher risk of MS (like those carrying HLA-DR15, those with detectable LMP1, or those with anti-ANO2 antibodies). It also strengthens the argument for EBV vaccination for MS prevention — and for earlier treatment of MS (which we know improves outcomes).
2. A new concept in cancer therapy improves survival
When I was doing pre-clinical cancer research, we spent a lot of time trying to find new agents to boost the efficacy of chemotherapy. Leucovorin is the only drug (to my knowledge) approved for this purpose, though, which boosts the efficacy of fluorouracil chemotherapy.
Corcept therapeutics might have a second: they’re working on glucocorticoid receptor blockers, which inhibit cortisol signalling. It’s been known for a while that this boosts chemotherapy in mice, by modulating the expression of apoptosis genes in cancer cells — but what about in humans?
Corcept showed that, in patients with ovarian cancer (resistant to previous lines of chemotherapy), combining the glucocorticoid receptor blocker Relacorilant with the standard of care chemotherapy (nab-paclitaxel) boosts chemotherapy efficacy — and, very impressively, increases overall survival:
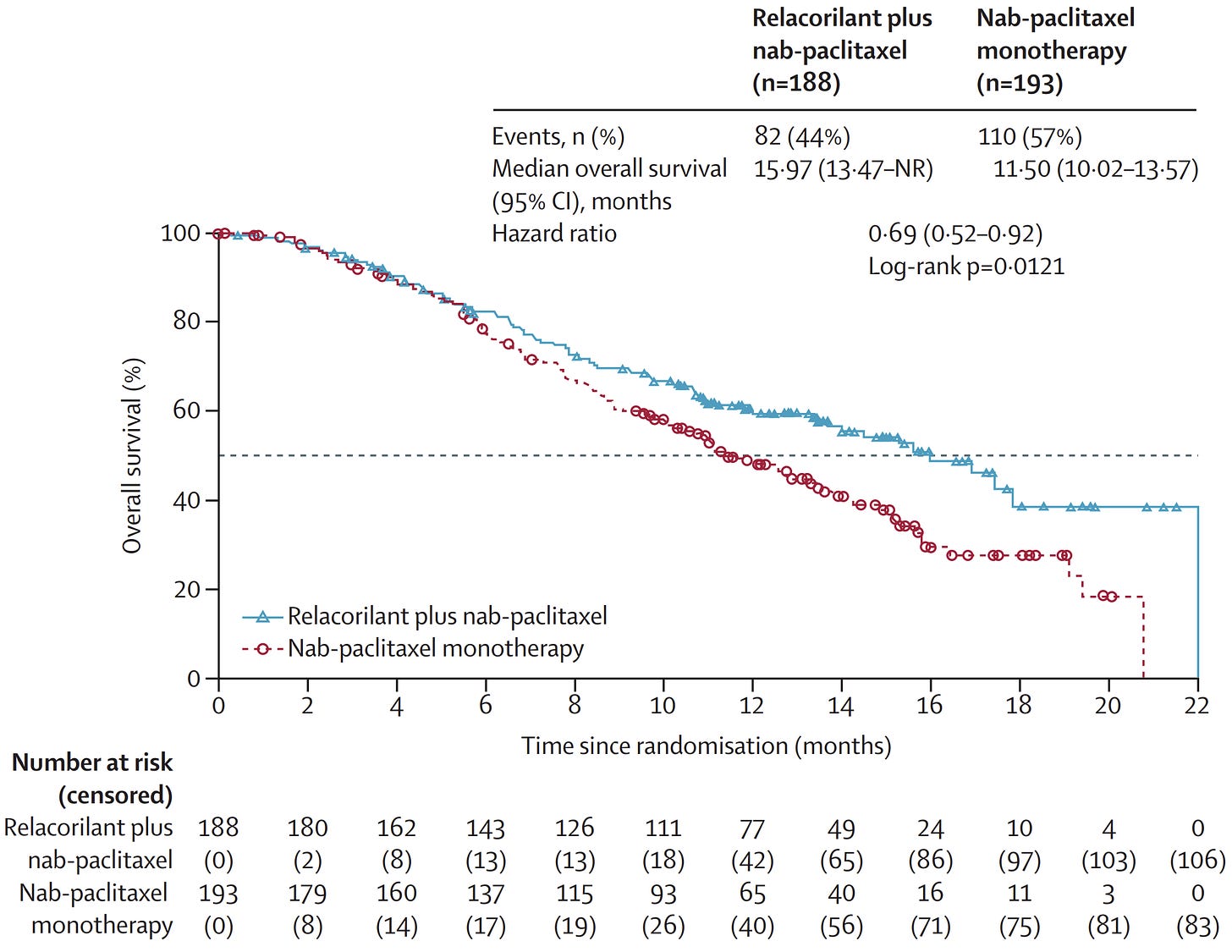
These data above are from the interim trial readout, which has now finished and met its primary endpoint this week. This approach could, in theory, generalise to other chemotherapy backbones, other cancer types, or immunotherapies too (and Corcept are pursuing each of these).
3. The FDA approves a liquid biopsy test for a cancer mutation
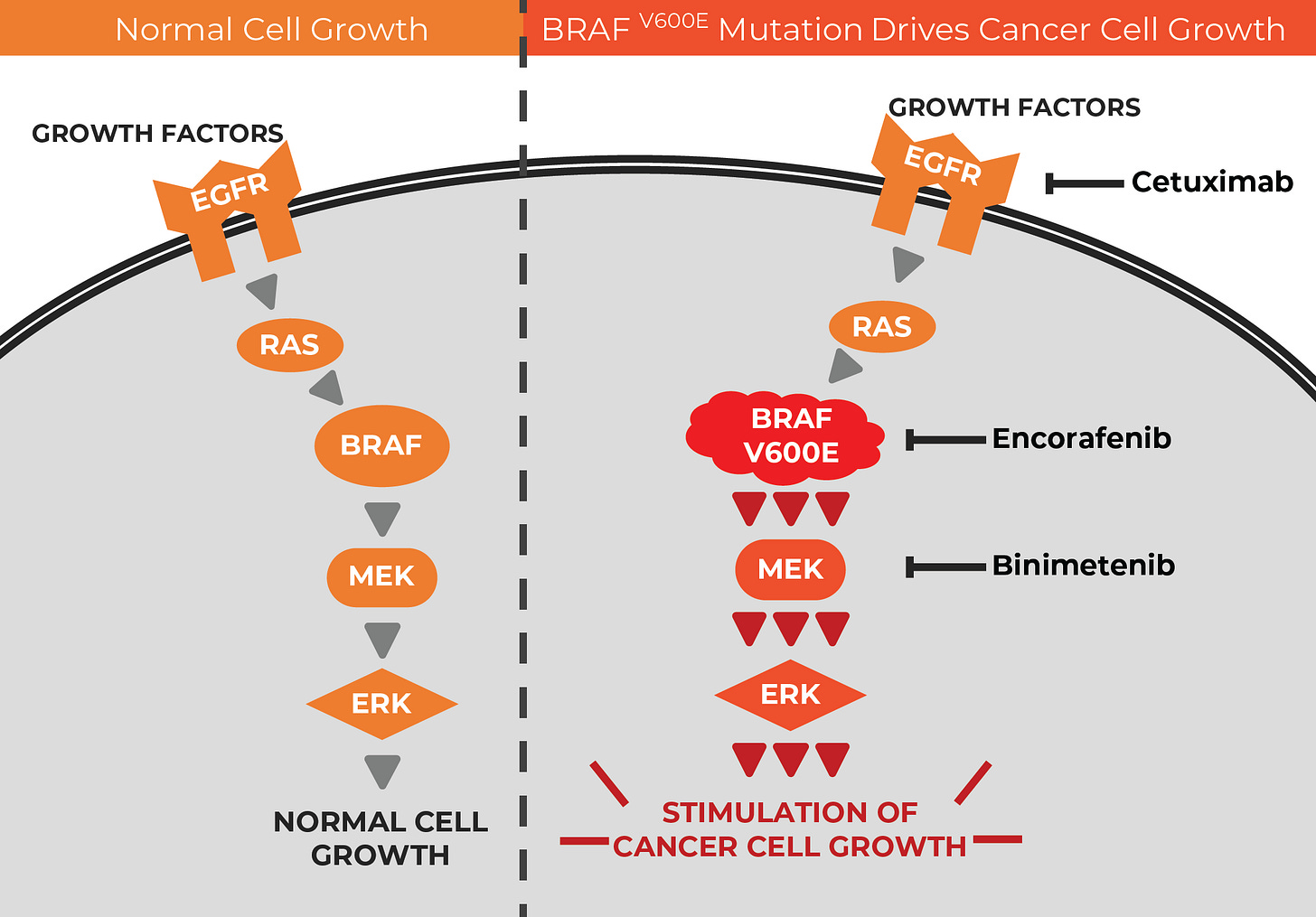
Last year a major trial found that a precision medicine approach — combined EGFR inhibition (with Cetuximab) and BRAF V600E inhibition (with Encorafenib), when added to chemotherapy, could beat standard care in patients with colorectal cancer and BRAF V600E mutations.
The question is: how can patients with this mutation be identified (fast), so they can benefit from the therapy?
Biopsy is imperfect for detecting the BRAF mutation — unavailable, insufficient, slow, or impacted by tumour heterogeneity — which is where this liquid biopsy (a blood test, that looks at circulating tumour DNA) comes in: it’s called Guardant360, and it was FDA-approved this week.
To explain the science here a little, Encorafenib inhibits the V600E mutation driving the tumours, and Cetuximab is used to anticipate the most common resistance mechanism, EGFR amplification:
4. A cancer vaccine improves survival in melanoma
I am a cancer vaccine enthusiast, but it can’t be ignored that a big trial in (arguably) the easiest cancer to produce a vaccine against, melanoma, did not reach statistical significance. These are the data — it’s for Moderna’s personalized mRNA vaccine combined with the PD1 inhibitor, pembrolizumab, in high-risk resected melanoma: